Performance bias occurs when there is unequal care between study groups.
This can happen in 2 scenarios:
- If researchers provided, intentionally or unintentionally, unequal treatment/care to different groups in the study
- If patients in different groups behaved differently
Performance bias affects the study validity since the observed outcome can now be attributed either:
- To the exposure/treatment
- Or to the unequal care between groups
Performance bias alters the study results unpredictably, so it is hard to control it analytically. It can affect randomized controlled trials as well as observational studies.
Example of performance bias
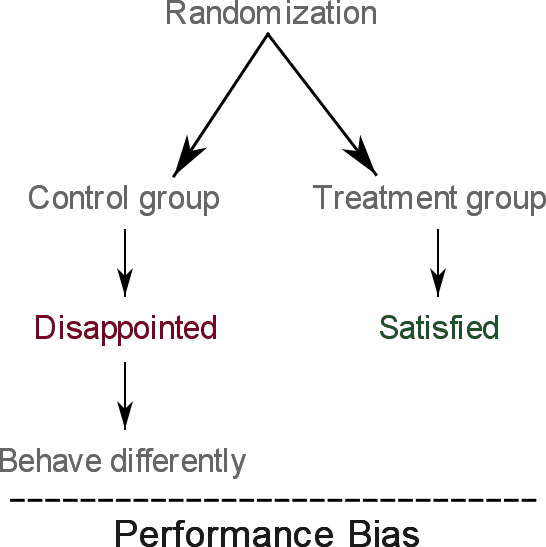
A qualitative study was conducted to examine the reaction of overweight participants on being randomized to either:
- Receive over 12 months of weight loss training done by experts (this is the treatment group)
- Or receive the usual care provided by general practitioners (this is the control group)
As participants in the control group were disappointed for being offered usual care instead of the new helpful program:
The study concluded that their reaction to disappointment may introduce performance bias.
The figure below summarizes this example:
How to avoid performance bias
Here are 2 solutions to avoid performance bias:
1. Blinding
Perhaps the simplest solution is to make participants and/or clinicians (or anyone involved in administering the treatment) unaware of which group received which treatment.
This is referred to as blinding.
Single blind: Refers to making patients unaware of the treatment they receive.
Double blind: Refers to making patients AND clinicians unaware of the treatment they receive/provide.
When possible, a double blind approach will always be preferred.
However, blinding is NOT always feasible, for instance:
- When conducting studies involving surgery: As it is impossible to blind participants to whether or not they received surgery
- In retrospective observational studies: Because in this case the treatment/exposure happened before the study even began (therefore it is impossible to blind people to what they already received)
In these cases, we will need to consider another measure to avoid performance bias.
2. Cluster stratification
I will explain cluster stratification with an example:
Suppose we want to run a clinical trial to study laparoscopic versus open surgery.
Because some technical variability may exist between surgeons (e.g. due to different levels of experience), our patients may experience unequal care which results in performance bias.
In this case, blinding isn’t going to solve our problem.
Instead we can use cluster stratification, which involves putting all patients who had an operation by a given surgeon in the SAME group (instead of randomizing individual patients).
This will allow surgeons to perform only 1 type of surgery: That which they are most comfortable with.
Therefore, cluster stratification will be measuring the benefits of the procedure more so than the difference in experience between surgeons, thereby minimizing performance bias. [Source]
Note 1: Does randomization prevent performance bias?
Randomization minimizes the difference between the treated and the control groups at the start of the trial. However, it does not prevent differential treatment of the groups later on. [Source]
Note 2: How to report performance bias?
It is not enough to mention in the limitations section that your study may be subject to performance bias, as it would be rarely helpful not to discuss or quantify the risk of having this bias. According to the newer version of the Cochrane Collaboration’s tool for assessing risk of bias in randomized trials, the risk of performance bias should be reported as low, high or unclear risk.