A case report is the description of the clinical story of a single patient, whereas a case-control study compares 2 groups of participants differing in outcome in order to determine if a suspected exposure in their past caused that difference.
| Case Report | Case-Control Study | |
|---|---|---|
| Participants involved | A case report describes the medical case of 1 particular patient | A case-control study requires 2 groups: 1. Cases: a group of participants who have the outcome (eg. diseased individuals) 2. Controls: a group of participants who do not have the outcome (eg. nondiseased individuals) |
| Goal | To report an interesting or unusual case of a patient | To look into the past records of these cases and controls in order to determine if the development of the outcome (eg. disease) has to do with an exposure to some risk factor |
| Study type | Observational (because the researcher only observes and describes the patient’s case and does not manipulate or control the events) | Observational (because the researcher does not influence who gets the exposure and who doesn’t) |
| Follow-up over time | Yes, sometimes the case report involves following the patient over a period of time | No, a case-control study is retrospective, meaning that it looks backwards in time to collect information about exposures that happened in the past |
| Example | In 1991, Fred Kern, Jr. reported the case of an 88-year-old man who has been eating 20-30 eggs each day for almost 15 years. The man had a normal cholesterol level as his body adapted to his unusual diet. [Source] | In 1993, Brent et al. compared 67 cases of adolescent suicide with 67 controls (adolescents who were demographically similar to the cases but did not commit suicide) and found that depression, bipolar disorder, substance abuse and conduct disorder were important predictors of adolescent suicide. [Source] |
| Advantages | Simple and inexpensive: as it involves following 1 patient only | – Fast to conduct: because participants are not followed over time – Useful for studying rare diseases: unlike other designs where we wait for participants to develop the disease, a case-control study works retrospectively: first the cases are chosen, then data about the exposure is collected |
| Limitations | Is considered a weak design because: – It represents a single story that does not always generalize to other cases. – Conclusions based on case reports may be biased (because the observed patient is not chosen at random from the population) or confounded by some unmeasured factors. | Is a weak design for assessing a causal relationship between exposure and outcome because of: – Selection bias: the selection of a control group is a complicated task that can easily go wrong and bias the study. – Recall bias: as the study depends on the memory of the participants to collect information about their exposure. |
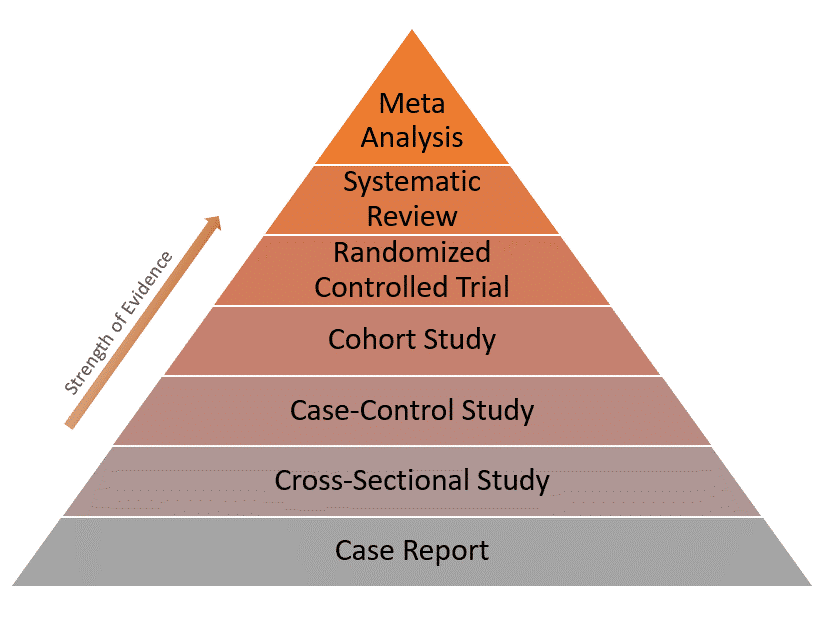
| Level of evidence | Has the lowest level of evidence of all study designs | A case-control design provides better evidence than the case report (see the evidence pyramid below) |
Here’s the evidence pyramid showing the level of evidence for different study designs: